elisa test labcorp|b burgdorferi test LabCorp : wholesalers Labcorp test details for Heparin-induced Platelet Antibody (HIPA) Skip to main content Close Menu. Logins. Individuals & Patients. Find a Lab View Test . Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004 Dec, 2(12):2133-2137. 15613017 . 9. Refaai MA, Laposata M .
Refurbished Used Autoclaves For Sale. Over the past twenty-five years, Booth Medical has .If chemotherapy waste or pathological waste was merely sterilized in an autoclave, it would still be hazardous and unsafe for landfill disposal. Incineration ensures these kinds of waste no longer pose any health risks to people, animals, or the environment.
{plog:ftitle_list}
No matter the setting or application an autoclave is used for, most models rely on a combination of temperature, pressure, steam, and time to achieve sterilization. Each of these .
ELISA (enzyme-linked immunosorbent assay) is an immunological assay designed for detecting and quantifying soluble substances – such as peptides, proteins, antibodies and hormones – .This panel utilizes FDA-cleared assays following the modified 2-tier testing (MTTT) algorithm to aid in the diagnosis of Lyme disease in individuals with clinical signs and symptoms consistent .Labcorp test details for Candida Antibodies, IgM, ELISA. Skip to main content Close Menu. Logins. Individuals & Patients. Find a Lab View Test . (ELISA) Reference Interval. Negative. LOINC® Map. Order Code Order Code Name Order Loinc Result Code Result Code Name UofM Result LOINC; 163010: Candida Antibodies IgM: 27391-2:This test is a preferred screening test for patients suspected to have bullous pemphigoid and its variants. The antibody levels can be used to monitor the effectiveness of drug treatment. The diverse spectrum of pemphigoid diseases is characterized by the formation of subepidermal blisters, which can develop in both the skin and mucous membranes.
This test was developed and its performance characteristics determined by Labcorp. It has not been cleared or approved by the Food and Drug Administration. Methodology. Real-time polymerase chain reaction (PCR) LOINC® Map. Order .Labcorp test details for Heparin-induced Platelet Antibody (HIPA) Skip to main content Close Menu. Logins. Individuals & Patients. Find a Lab View Test . Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004 Dec, 2(12):2133-2137. 15613017 . 9. Refaai MA, Laposata M .
This test is used for the quantitative measure of HER-2/neu in human serum. HER-2/neu values obtained may be used in the follow-up and monitoring of patients with metastatic breast cancer. HER-2/neu values should be used in conjunction with information available from clinical and other diagnostic procedures in the management of breast cancer. The clinical utility of the .Four hundred thirty-three samples routinely submitted for NMO-Ab testing were tested by both the aquaporin-4 autoantibody ELISA and indirect immunofluorescence assay (IFA) performed at Mayo Clinic Laboratory. 8 98.1% of results were in agreement with a positive result agreement of 78.6% and negative result agreement of 98.8% (IFA as reference .
lyme disease line blot LabCorp
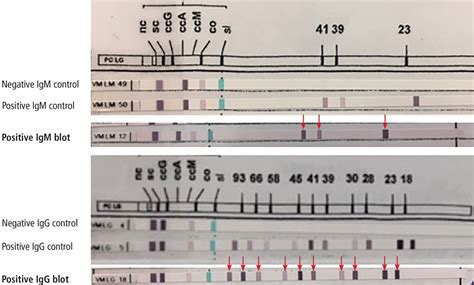
This test is used for evaluation of patients with a history of, or suspected, tick exposure who are presenting with fever, myalgia, headache, nausea and other nonspecific symptoms. . IgG and IgM [138315]: This test was developed and its performance characteristics determined by Labcorp. It has not been cleared or approved by the Food and Drug .Labcorp test details for Antimyeloperoxidase (MPO) Antibodies. 2 - 4 days. Turnaround time is defined as the usual number of days from the date of pickup of a specimen for testing to when the result is released to the ordering provider.The quantitative allergen-specific IgE test is indicated (1) to determine whether an individual has elevated allergen-specific IgE antibodies; (2) if specific allergic sensitivity is needed to allow immunotherapy to be initiated; (3) when testing individuals for agents that may potentially cause anaphylaxis; (4) when evaluating individuals who .
Labcorp test details for Anti-CCP (Cyclic Citrullinated Peptide) Antibodies, IgG and IgA (RDL) . (ELISA) Footnotes. 1. Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003 Sep;62(9):870-874. 12922961. 2. Maksymowych WP, Naides SJ, Bykerk V, et al. Serum 14-3-3 eta is a novel marker .
If protozoal, filarial, or trypanosomal infection is strongly suspected, test should be performed at least three times with samples obtained at different times in the fever cycle. Methodology Wright stain; microscopic examination of thick and thin peripheral blood smears stained with Romanovsky dye (in particular Giemsa).Qualitative detection of antibodies against the fungus Coccidioides to aid in the diagnosis of Coccidioidomycosis (Valley Fever). Specimens positive for Coccidioides antibody by EIA should be confirmed by additional testing (complement fixation and immunodiffusion).Coccidioides live in the dust and soil in some areas of the southwestern United States, Mexico, and South America. The CDC Zika MAC-ELISA Test Has Received FDA Emergency Use Authorization and Complements the RealStar ® Zika Virus RT-PCR Kit U.S.. BURLINGTON, N.C.--(BUSINESS WIRE)--Aug. 2, 2016-- Laboratory Corporation of America ® Holdings (LabCorp ®) (NYSE:LH) today announced the nationwide availability of testing for Zika virus using the Zika .The anti-PLA2R ELISA (IgG) test kit is intended for the qualitative or semiquantitative determination of IgG class autoantibodies against phospholipase A2 receptor (PLA2R) in human serum. 1 It is used as an aid in the diagnosis of primary membranous glomerulonephritis (pMGN), in conjunction with other laboratory and clinical findings.
Labcorp test details for Echinococcus Antibody. 1 - 7 days. Turnaround time is defined as the usual number of days from the date of pickup of a specimen for testing to when the result is released to the ordering provider.Labcorp test details for Antiskin Autoantibodies, Quantitative. 2 - 7 days. Turnaround time is defined as the usual number of days from the date of pickup of a specimen for testing to when the result is released to the ordering provider.Labcorp test details for IA2 Autoantibodies (Endocrine Sciences . please submit separate frozen specimens for each test requested. Storage Instructions. Freeze. Stability Requirements. . Freeze/thaw cycles. Stable x6. Test Details. Methodology. Enzyme-linked Immunosorbent Assay (ELISA) Additional Information. Type 1 diabetes, commonly .
Labcorp test details for Anti-La (SS-B) Ab (RDL) 5 - 7 days. Turnaround time is defined as the usual number of days from the date of pickup of a specimen for testing to when the result is released to the ordering provider.In the past, it was considered unnecessary to test for anti-DNA in patients with a negative test for antinuclear antibodies. A group of “ANA-negative lupus” patients has been described with anti-ssDNA and anti-SS-A/Ro and anti-SS-B/La; however, HEp-2 substrate is much more sensitive than frozen section substrates, and it is uncommon for . Assay expands company's leadership in precision medicine and cell and gene therapy. BURLINGTON, N.C., April 29, 2024 /PRNewswire/ -- Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, today announced the U.S. Food and Drug Administration (FDA) has approved its nAbCyte™ Anti-AAVRh74var HB-FE Assay, a .
This test can be used for the diagnosis or the exclusion of exocrine pancreatic insufficiency, which may be associated with chronic pancreatitis, cystic fibrosis, carcinoma of the pancreas, diabetes mellitus type 1 (insulin-dependent diabetes mellitus), Shwachman-Diamond syndrome and other etiologies of pancreatic insufficiency.
Although the available research is too limited to address biomarker accuracy in ruling out significant and advanced fibrosis in patients with NAFLD among the general population, the estimations based on one study suggest that the ELF™ test has a high NPV when the prevalence is lower than 30%. 3 This highlights the value of the ELF™ test as .
Order Code Order Code Name Order Loinc Result Code Result Code Name UofM Result LOINC; 164000: Strongyloides IgG Antibody: 80660-4: 164001: Strongyloides IgG AntibodyPatients diagnosed with chronic granulomatous disease and/or Job's syndrome may yield a reduced detection of galactomannan. 1 Reduced assay sensitivity may occur in patients receiving concomitant antifungal therapy. 2 Penicillium species, Alternaria species, Paecilomyces species, Geotrichum species, and Histoplasma species have demonstrated reactivity with the .This test allows for the detection of the presence of antibodies to glutamic acid decarboxylase, which provides early evidence of autoimmune disease activity; its measurement has been shown to be useful in assisting the physician in the prediction, diagnosis, and management of patients with diabetes. 2-6 Glutamic acid decarboxylase (GAD 65) is an enzyme that is produced .
The QuantiFERON TB test has been shown to be accurate in HIV-positive individuals with moderately advanced disease, but in the severely immunocompromised the test may be impaired by T-cell anergy. 2,3 Active TB disease may result in a negative test as reduction of in vitro IFN-γ release has been described and may be due to suppressive .At Labcorp, our ANA tests are performed by immunofluorescence assay (IFA) Gold standard The American College of Rheumatology . Sato EI, Rodrigues SH, et al. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases.
b burgdorferi test LabCorp
xtip ranin pipettes
LabCorp red top tubes
Autoclave in a pyrex bottle. Store in the fridge and use at room temperature, near the flame. Casamino acids 10%. Dissolve 10 g of powder in approximately 80 ml of deionized .How to Use Your CASTLE 777" SPEED-CLAVE Step by step operating procedure This manual was restored and digitized by Jacko [email protected] 05/20/2015
elisa test labcorp|b burgdorferi test LabCorp